
From ADC to XDC: Wave Surge, Innovation Far Away
Release time:
2024-09-29 17:33
Source:
Antibody-drug conjugates (Antibody-drug Conjugates,ADCs) are currently one of the most active innovative drug tracks. Since 2022, there have been more than 140 global licensing collaborations and acquisitions around ADC drugs, with a cumulative total of more than $150 billion in potential transactions. In the cold winter of medicine, the ADC track bucked the trend and made great progress all the way.
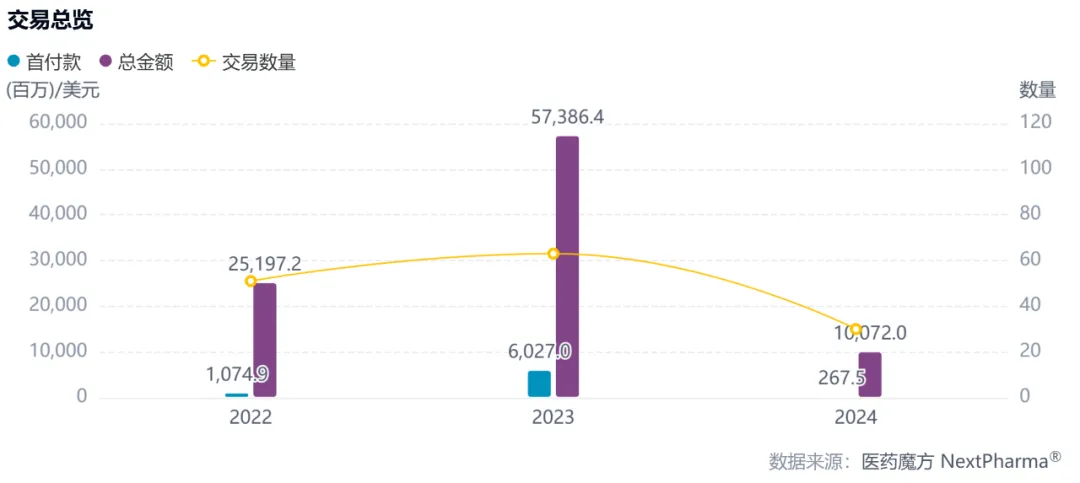
2022-01-01 to 2024-09-25, Overview of ADC Transactions (Excluding Corporate Mergers and Acquisitions)
ADC is composed of three key components: antibody, linker and payload, which is known as the "magic bullet" of cancer treatment because of its combination of tumor targeting properties of antibodies and the killing of cytotoxic drugs ". Since the first drug was approved in 2000, 15 ADC products have been approved worldwide. The current global ADC pipeline is huge, with more than 1000 ADC projects, of which more than 300 ADC molecules are active in various stages of clinical trials.
Such prosperity is inextricably linked to the potential of ADC drugs to treat diseases. Currently, there are 15 ADC products on the market, including a variety of blood cancers and solid tumors. It is worth mentioning that Enhertu(HER2 ADC), as a key force in the ADC industry boom, was just approved in April this year for the treatment of adult patients with unresectable or metastatic HER2 positive (IHC 3) solid tumors who have received systemic treatment in the past and have no satisfactory alternative treatment. This is the first ADC therapy with unlimited cancer indications, revealing the huge imagination of ADC drugs in the field of anti-cancer. At the same time, in addition to cancer, ADC drugs are also expanding into autoimmune diseases, viral infections, eye diseases and rare diseases.
In terms of commercial performance, ADC drugs such as Adcetris, Kadcyla, Padcev, Enhertu and Trodelvy have entered the "blockbuster drug" club. Among them, Enhertu became the best-selling ADC drug in 2023 with sales of $2.566 billion. Thanks to the continued growth in sales of a number of drugs, the global ADC drug market exceeded the $10 billion mark for the first time in 2023. According to an analysis released by Nature Reviews Drug Discovery magazine in April this year, ADC drug revenue is expected to reach $26 billion by 2028.
The continued popularity of the bioconjugate drug track, represented by ADC, has also driven the rapid development of the corresponding CRO/CRDMO industry, and the related outsourcing services market has continued to grow over the past few years. According to Frost & Sullivan's analysis, the global ADC outsourcing services market is worth $1.5 billion in 2022, and the global ADC outsourcing services market is expected to expand significantly to $11 billion by 2030.
Yao Ming United is a global leader in CRDMO, a bioconjugate drug. Since its establishment in 2021, it has delivered a bright report card every year. According to the company's 2024H1 financial report, by June 30, 2024, the total number of customers in the world has accumulated to 419, and 13 of the TOP20 large pharmaceutical companies have reached different stages of project cooperation with the drug. As of the first half of 2024, the company has achieved the milestone of cumulative successful delivery of more than 11,000 bioconjugate molecules, helping customers submit 71 ADC drug candidate IND applications worldwide.
Different from traditional small molecules and macromolecules, what are the barriers of CRDMO focusing on ADC and other bioconjugate drugs? What technological innovations and service innovations have been made in the past three years? Will the industry wave of ADC and XDC continue in the next few years?On September 11, at the scene of the 3rd Global Innovation Summit on Bioconjugate Drugs (2024 Global XDC Innovation Conference), Medical Rubik's Cube interviewed Dr. Li Jincai, CEO of Yao Minghe United, and Dr. Zhu Meiying, CTO, on the above issues.
ADC track erupts "with traces to follow"
WuXi XDC (WuXi XDC) officially started in May 2021, and we can see a thing or two from the development trajectory of the ADC track as why we chose to use bioconjugate drugs as the new business power point of the drug at such a point in time. Although the first ADC was approved for listing in 2000, due to the unsuccessful drug research and development in the following 10 years, the ADC track has not yet formed a scale by around 2010. Since 2017, new ADC drugs have been on the market, and since 2019, three new ADC drugs have been approved each year for three consecutive years, and the growth trend of the ADC industry has become increasingly prominent.
Yao Ming Bio undertook the first ADC project in 2012 and is one of the first companies in the world to start ADC outsourcing services. In the years since then, we have seen the iteration of ADC technology and the latest progress of the track through continuous service to ADC customers. Based on a forward-looking judgment of industry trends, combined with the industry challenges and pain points seen in the process of serving customers, around 2020, the company began to consider the integration of ADC and other bioconjugate drug outsourcing services. In 2021, WuXi XDC (WuXi XDC) was born. After that, everyone saw the company's proven strengths, focused on the industry track, invested with the greatest efforts, and provided the support of funds, teams and other supporting resources. This is also the reason why WuXi XDC can develop into the global leader of CRDMO, a bioconjugate drug, in such a short time.

Dr. Li Jincai, CEO of Yao Ming United
According to Dr. Li Jincai's introduction at the Global XDC 2024 Summit, compared with traditional small molecules and macromolecules outsourcing services, it is relatively more difficult to be a CRDMO that focuses on ADC and other bioconjugate drugs. ADC research and development spans large molecules and small molecules. Traditionally, the two drugs are separated. Even in the same company, large molecules and small molecules are two relatively independent teams. ADC research and development requires teams in these two directions to work together, and it is not easy to do this kind of interdisciplinary and cross-domain integration. Because macromolecules, small molecules, whether it is research and development, production or quality system, are very different. In this context, with the same quality system as the standard, the barriers are very high, but it is very helpful to customers, and efficient quality management can be carried out. At the same time, the process chain is simplified and the project development cycle is greatly shortened.

Dr. Zhu Meiying, CTO of Yao Ming United
"There are several layers to ADC development challenges, including how to design the ADC, how to perform the analysis after ADC is obtained, and how to remove impurities. In addition, due to the highly complex structure of bioconjugate drugs such as ADCs, process development and production are extremely challenging in the process of industrialization. The CMC for ADCs needs to bring together professionals from different disciplines." Dr. Zhu Meiying added.
Despite the challenges, thanks to the strong management system and all-round multidisciplinary collaboration capabilities of the company, the company has been ableDevelopment time of antibody DNA sequence to new drug clinical trial application (IND)A significant reduction of 13 to 15 months, equivalent to half of the industry's regular development time. In addition, the drug combination is also currentlyVery few people in the world can be one-stop in the same park.Companies that have completed the development of antibody intermediates, load linkers, coupling stock solutions, coupling preparations, and GMP production.
It is worth mentioning that in the past three years or so, the drug minghe union has made continuous breakthroughs around the two dimensions of technological innovation and capacity expansion. At the Global XDC 2024 Summit, the Medical Rubik's Cube learned that in the development of innovative coupling technologies, the Pharmaceutical Minghe Union has independently developedWuXiDAR4TMtechnology platform. This technology can increase the ADC with a DAR of 4 from about 40% to more than 70% without engineering the antibody or adding any enzymes. This allows WuXiDAR4 ADCs to exhibit better in vivo potency and more stable pharmacokinetic (PK) properties. Clinical data suggest that WuXiDAR4 ADCs have shown better tolerability in clinical trials. At present, seven ADCs using WuXiDAR4 technology have entered the clinical stage.
In terms of production capacity, in order to meet the strong and growing demand of the global industry, Yao Ming United launched its first self-designed mAb/DS dual-function production line (BCM2 L1) in September 2023 and successfully started GMP production, providing end-to-end production from antibody intermediates to bioconjugate drug stock solutions. At the same time, the company will further expand its production capacity in Wuxi, and the new mAb/DS dual-function production line (BCM2 L2) will be put into operation in the fourth quarter of 2024. The bioconjugate production line XDP3 is under construction and is expected to be operational in the second quarter of 2025. In addition, the company's integrated production site in Singapore broke ground in March 2024 and is currently progressing smoothly and is expected to be operational by the end of 2025/early 2026.
Dr. Lee Kam-choi said: "The Medicine Ming JointThe two things we always insist on doing are "continuous investment in technology" and "continuous expansion of capabilities'. We have always regarded technology as the core differentiation advantage. How ADC technology will iterate in the future, what new challenges and characteristics will evolve from ADC to XDC, what new technologies and new trends will emerge in the future, and what capabilities, resources and talent teams CRDMO companies need to match to deal with this change. The company will always pay attention to these issues and make a forward-looking layout."
"In terms of capacity development, one of the key directions is 'capacity '. The shortage of ADC capacity has always been a common challenge faced by the global industry. Because capacity expansion has the characteristics of long-term and heavy capital, in a fast-growing industry, it seems that capacity is always" unable to keep up'. In the next few years, in addition to the current capacity layout, the Pharmaceutical Federation will further make dynamic judgments and decisions based on the overall trend of industry development. We hope to meet the growing industry demand and accelerate the development and commercialization of innovative ADC therapies through continuous deep cultivation of technology platforms and capacity construction."
XDC Ride the Wind
In the past few years, the wave of research and development of bioconjugate drugs such as ADC has swept the world. As for the origin of this wave, Dr. Zhu Meiying, who has been deeply engaged in ADC track for 15 years, told the Medical Rubik's Cube that after the approval of Kadcyla(T-DM1) in 2013, no new ADC drug has been approved for 3 consecutive years, and several products have failed in Phase III clinical trials. The industry has also questioned the ADC. The approval of Enhertu(DS-8201) really gives the industry new hope. One is effective in solid tumors, especially the results of the unresectable and/or metastatic breast cancer (mBC) with low HER2 expression, and the results of the head-to-head study with Kadcyla(T-DM1). This tangible benefit to patients is a big part of driving the ADC wave. Second, Enhertu has brought a breakthrough in ADC design and adopted a less toxic payload, which greatly enhanced the industry's imagination of ADC, and the idea was suddenly opened. This is recognized as a turning point in the development of the ADC circuit.
ADC is composed of multiple components, so there are many innovations. Different targets can be developed, and different "target heads" (such as monoclonal antibodies, biantibodies, peptides, etc.) can also be developed. At the same time, there are many kinds of linker, coupling technology also keeps pace with the times, and the continuous enrichment of load types, the combination of innovations in these different dimensions is endless.
"The world's first ADC was approved in 2000, 24 years later, and the ADC has endured for a long time. I believe that the space for exploration in the future is very broad, and this wave of innovation will continue and translate into medical breakthroughs for the benefit of patients around the world." Dr. Zhu Meiying said.
With the explosive growth of the ADC track, the bioconjugate drug (XDC) track is riding the wind, and the curtain of the era of "everything can be coupled" is opening. In the past few years, a variety of new biological coupling technology has made rapid development, opening up a new path of drug research and development. For example, antibody-oligonucleotide conjugates (AOC) Using the high targeting of antibodies, oligonucleotides are precisely delivered to specific cells or tissues to achieve gene regulation or therapeutic purposes. AOC technology is expected to solve the difficulties of oligonucleotide delivery and short half-life, and bring new breakthroughs for the treatment of various diseases. In addition, the degrader-antibody conjugate (DAC), radionuclide-drug conjugates (RDC) and other technologies also show a wide range of application prospects in the fields of tumor treatment, and are one of the hotspots of current biomedical research.
In the global wave of life science innovation, the bioconjugate drug industry is ushering in unprecedented development opportunities, with rapid technological changes and endless innovations. The Global XDC Summit can be said to be a window to witness the breakthrough of conjugate drug innovation. At this year's summit, players from around the world showed a large number of the latest scientific research achievements, including the research and development of new target ADC and the next generation ADC, the innovative design of new conjugate drugs, and the exploration of indications outside the tumor.
"Because we believe, we see-with this historic opportunity for development, we believe that the power of innovation from around the world will open a new chapter in the development of the bioconjugate drug industry. The drug will also continue to play the role of 'enabler', working with global partners to usher in an even more glorious future for the ADC and XDC circuit." dr lee kam-tsei looked ahead.
