
Daiichi Sankyo's TROP2 ADC has been approved for market launch in the United States.
Release time:
2025-01-20 20:42
Source:
On January 17 (local time in the U.S.), Daiichi Sankyo announced that Datroway® (datopotamab deruxtecan) has been approved for marketing in the U.S. for the treatment of adult patients with HR-positive, HER2-negative (IHC 0, IHC 1+, or IHC 2+/ISH-) unresectable or metastatic breast cancer who have previously received endocrine therapy and chemotherapy. The indication for Datroway® was approved in Japan on December 27, 2024, and registration applications in other countries/regions (EU, China, and others) are under review.This drug is the second antibody-drug conjugate approved in the U.S. using Daiichi Sankyo's DXd ADC technology platform, following Enhertu® (trastuzumab deruxtecan).

Datroway® is developed using Daiichi Sankyo's unique DXd ADC technology platform, consisting of a humanized anti-TROP2 IgG1 monoclonal antibody (developed in collaboration with Sapporo Medical University) linked via a cleavable tetrapeptide linker to multiple topoisomerase I inhibitor payloads (a derivate of irinotecan, DXd). This product is developed by Daiichi Sankyo and co-developed with AstraZeneca. In July 2020, Daiichi Sankyo and AstraZeneca entered into a global collaboration to jointly develop and commercialize Datroway® (datopotamab deruxtecan).
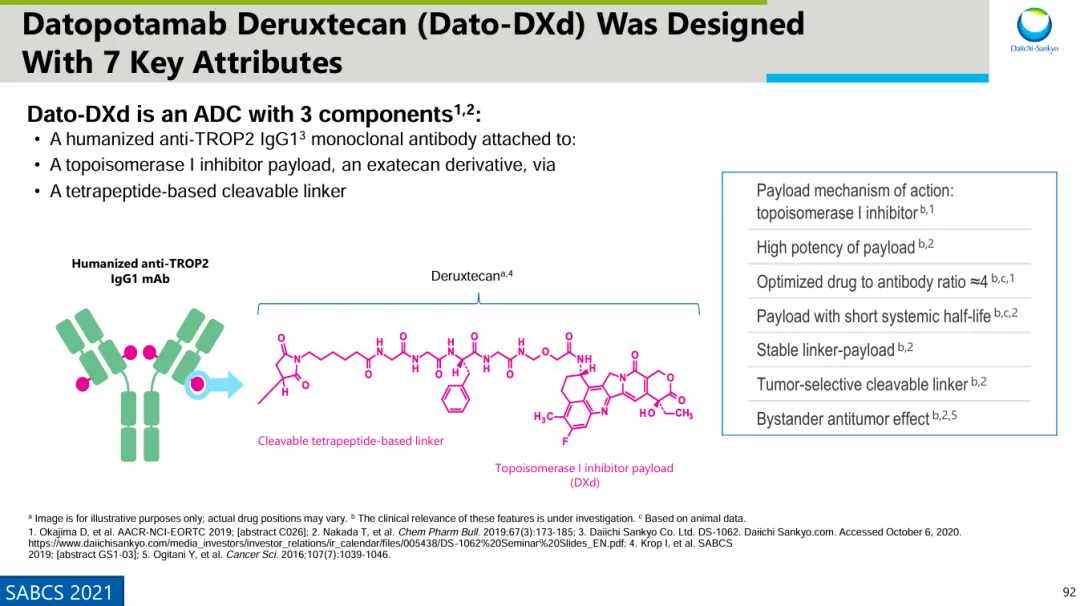
Source: Daiichi Sankyo SABCS 2021 Conference PPT
The FDA's approval of Datroway® is based on the results of the Phase III study TROPION-Breast01. TROPION-Breast01 is a global, randomized, multicenter, open-label Phase III study (n=732) that evaluated the efficacy and safety of Datroway® (6 mg/kg, administered intravenously every 21 days) compared to investigator-selected single-agent chemotherapy (eribulin, capecitabine, vinorelbine, or gemcitabine) in adult patients with HR-positive/HER2-negative (IHC 0, IHC 1+, or IHC 2+/ISH-) unresectable or metastatic breast cancer who experienced disease progression during endocrine therapy or were unsuitable for endocrine therapy and had previously received at least one line of chemotherapy. After disease progression or discontinuation of treatment with Datroway® or chemotherapy, patients could choose subsequent treatment based on the judgment of their attending physician, but cross-treatment between study groups was not allowed.
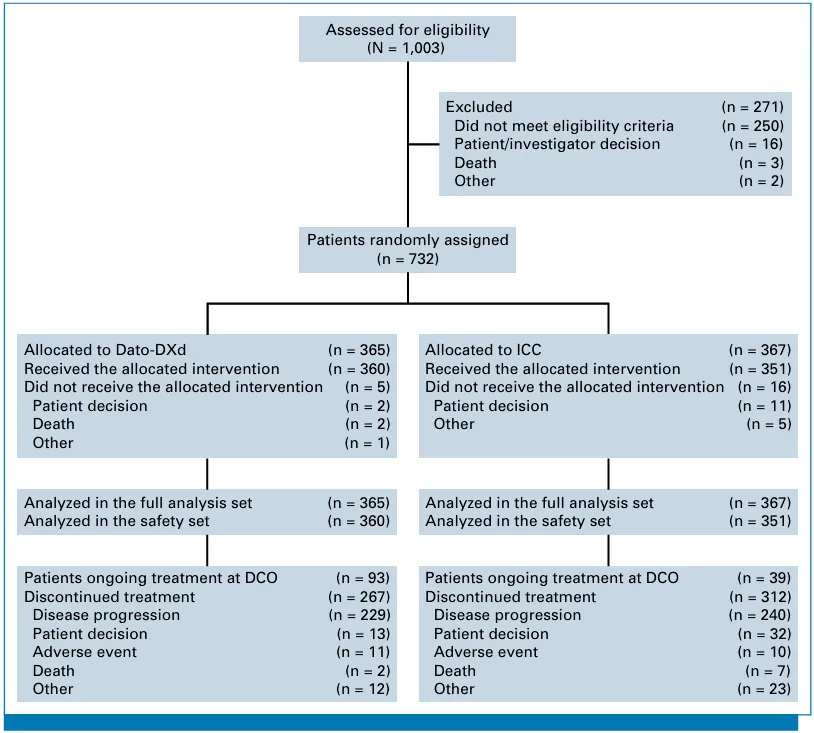
Source: Journal of Clinical Oncology
The dual primary endpoints of this study were progression-free survival (PFS) assessed by blinded independent central review (BICR) and overall survival (OS). Key secondary endpoints included objective response rate (ORR), duration of response (DoR), investigator-assessed PFS, disease control rate (DCR), time to first subsequent treatment, and safety.
In this study, compared to the investigator-selected chemotherapy group, the Datroway® group of HR-positive/HER2-negative metastatic breast cancer patientshad a significantly reduced risk of disease progression or death by 37%.(Hazard ratio [HR]=0.63; 95% confidence interval [CI]: 0.52–0.76; p<0.0001).The median PFS for patients in the Datroway® group was 6.9 months, while the median PFS for patients in the chemotherapy group was only 4.9 months.
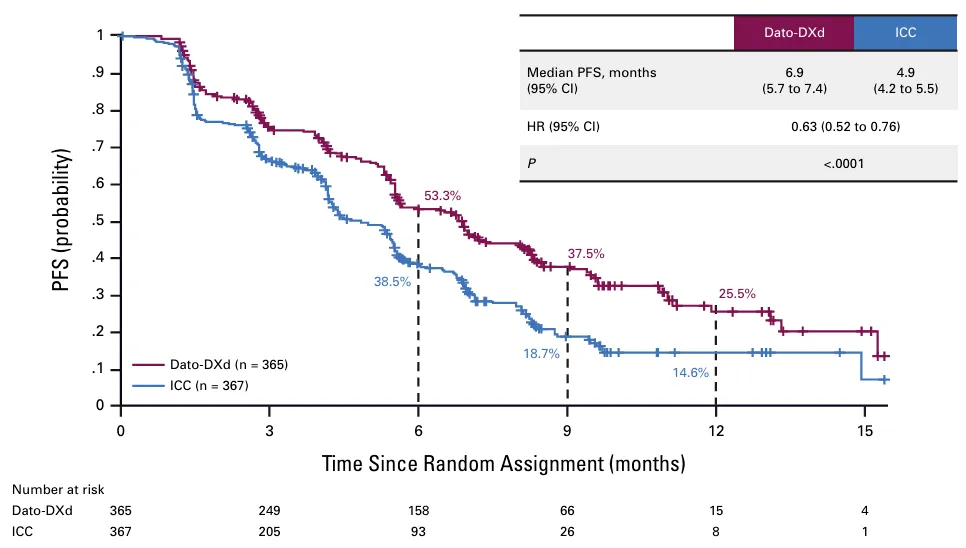
Source: Journal of Clinical Oncology
The confirmed ORR for the Datroway® treatment group was 36.4%, while the ORR for the chemotherapy group was 22.9%. The Datroway® group observed 2 cases (0.5%) of complete response (CR) and 131 cases (35.9%) of partial response (PR), while the chemotherapy group had 0 cases of CR and 84 cases (22.9%) of PR. The median DoR for the Datroway® group was 6.7 months (95% CI: 5.6–9.8), while the median DoR for the chemotherapy group was 5.7 months (95% CI: 4.9–6.8).
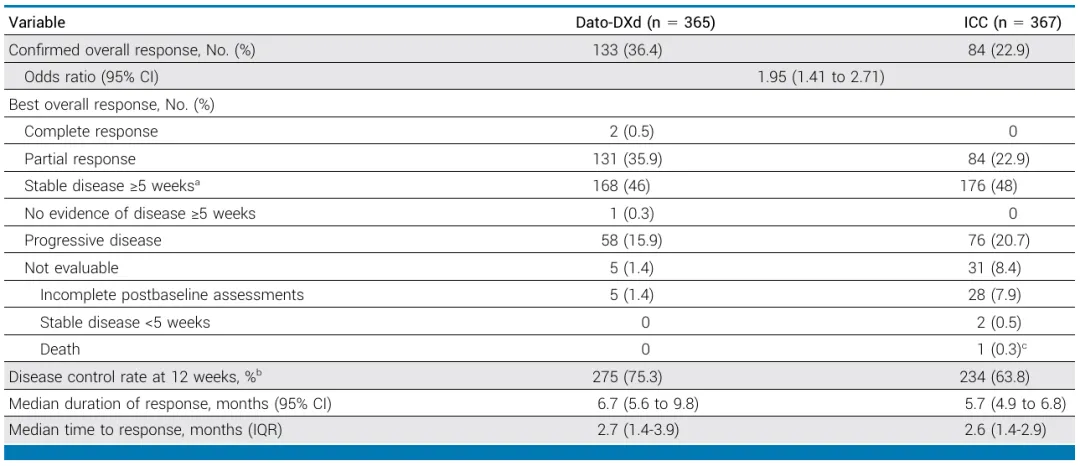
Source: Journal of Clinical Oncology
The TROPION-Breast01 study evaluated the safety of Datroway® (6 mg/kg) in 360 patients. The most common (>20%) adverse reactions (including laboratory abnormalities) were oral mucositis, nausea, fatigue, leukopenia, hypocalcemia, alopecia, lymphopenia, anemia, constipation, neutropenia, dry eye, elevated alanine aminotransferase, vomiting, elevated aspartate aminotransferase, elevated alkaline phosphatase, and keratitis. Serious adverse reactions included urinary tract infection (1.9%), COVID-19 infection (1.7%), interstitial lung disease (ILD)/non-infectious pneumonia (1.1%), acute kidney injury (0.6%), pulmonary embolism (0.6%), vomiting (0.6%), diarrhea (0.6%), mild hemiparesis (0.6%), and anemia (0.6%). One patient's death (0.3%) was attributed to an adverse reaction (ILD/non-infectious pneumonia).
Daiichi Sankyo and AstraZeneca have a comprehensive plan for the development of Datroway®, having initiated a series of global clinical development projects to evaluate the efficacy and safety of this drug in treating various cancers, including non-small cell lung cancer, triple-negative breast cancer, and HR-positive/HER2-negative breast cancer. Among these, there are 7 Phase III studies for lung cancer and 5 Phase III studies for breast cancer, aimed at evaluating the efficacy and safety of Datroway® as a monotherapy and in combination with other drugs for various cancers.
In 2022, there were over 300,000 new cases of breast cancer in the U.S. Although the survival rate for patients diagnosed with early breast cancer is high, it is estimated that only about 30% of patients diagnosed with metastatic disease or who progress to metastatic disease can survive for 5 years after diagnosis.
About 70% of diagnosed cases are long considered to be HR-positive, HER2-negative breast cancer (HER2 score of IHC 0, IHC 1+, or IHC 2+/ISH-). In the early treatment of HR-positive metastatic breast cancer, endocrine therapy is usually given continuously. However, the further efficacy of re-administering endocrine therapy after second-line endocrine therapy is often limited. Currently, the standard therapy after endocrine therapy is chemotherapy, but its response rate and prognosis are poor.
Targeting TROP2 drugs are currently considered an effective treatment strategy for HR-positive/HER2-negative breast cancer, and TROP2 ADC is the only biological therapy option available for these patients.The research and development competition in the TROP2 ADC field is becoming increasingly fierce, with three ADC drugs already approved globally: Trodelvy® (gogolizumab), Datroway® (datopotamab deruxtecan), and Jiatailai® (lukanumab). Among them, Jiatailai® has not yet been approved for HR-positive breast cancer indications, only for triple-negative breast cancer indications. In addition, there are three products in the global III clinical development stage waiting to join the competition, including SHR-A1921 (Hengrui Medicine), ESG401 (Shijian Bio), and FDA018 (Fudan Zhangjiang).
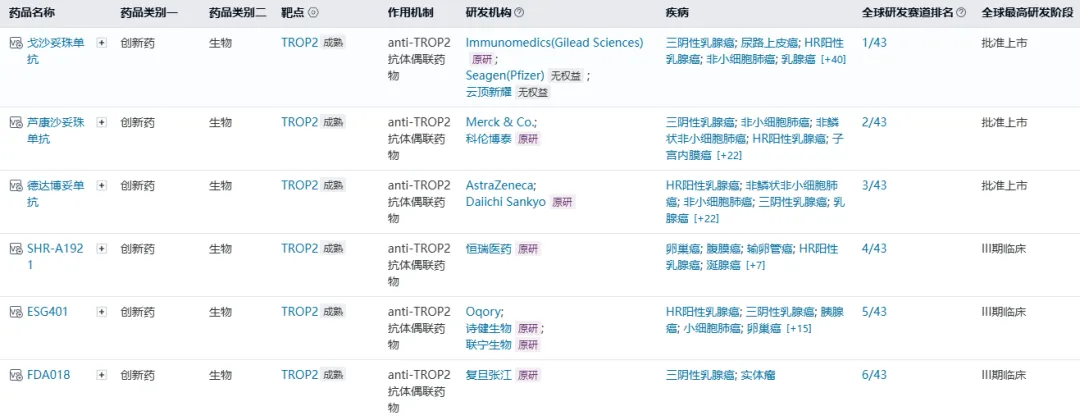
Source: Nextpharma database.
Dr. Aditya Bardia, Director of the Breast Cancer Research Program at the Jonsson Comprehensive Cancer Center at UCLA, and Global Principal Investigator of TROPION-Breast01, stated: "Although significant progress has been made in the treatment of HR-positive, HER2-negative metastatic breast cancer, new therapies are still needed to address disease progression after endocrine therapy and initial chemotherapy, a frequent and complex challenge. The approval of Dato-DXd (a novel targeted TROP2 ADC) provides new treatment options for patients with metastatic breast cancer."
Caitlin Lewis, Senior Vice President of Strategy and Mission at "Living Beyond Breast Cancer," stated, "Among patients with HR-positive, HER2-negative metastatic breast cancer, only one-third survive more than five years after diagnosis, highlighting the urgent need for more effective therapies. The approval of Datroway® is a significant advancement, providing new and urgently needed treatment options for patients with HR-positive metastatic breast cancer."
Ken Keller, Global Head of Oncology Business at Daiichi Sankyo, President and CEO, stated: "The approval of Datroway® provides new opportunities for HR-positive, HER2-negative breast cancer patients who have previously received endocrine therapy and chemotherapy, allowing these patients to receive timely treatment with the novel targeted TROP2 ADC during the metastatic stage. Datroway® is the latest member of our innovative cancer treatment portfolio and the fourth drug approved in the U.S. in our oncology drug development pipeline."
References
[1] American Cancer Society. Key Statistics for Breast Cancer. Accessed January 2025.
[2] National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer Subtypes. Accessed January 2025.
[3] Manohar P, et al. Cancer Biol Med. 2022 Feb 15; 19(2):202–212.
[4] Cortes J, et al. Lancet. 2011;377:914-923.
[5] Yuan P, et al. Eur J Cancer. 2019;112:57-65.
[6] Jerusalem G, et al. JAMA Oncol. 2018;4(10):1367–1374.
Previous Page
